Quantum Numbers - Chemistry LibreTexts
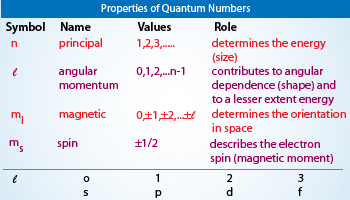
Introduction to the Aufbau Principle in Chemistry. Photons, a concept often applied to quanta with other sources of electromagnetic radiation such as X rays and gamma rays, are certain particle-like packets of light. The second character identifies the subshell. Additionally, this wave function for an electron within an atom is called the atomic orbital. If you attend a college or professional football game, you need a ticket to get in. However, some, usually called a parity , are multiplicative; i. The tally of quantum numbers varies from system to system and has no universal answer. Orbitals The number of orbitals in a subshell is equivalent to the number of values the magnetic quantum number ml takes on. In quantum physics and chemistry , quantum numbers describe values of conserved quantities in the dynamics of a quantum system.