Quantum Numbers and Rules – College Physics chapters
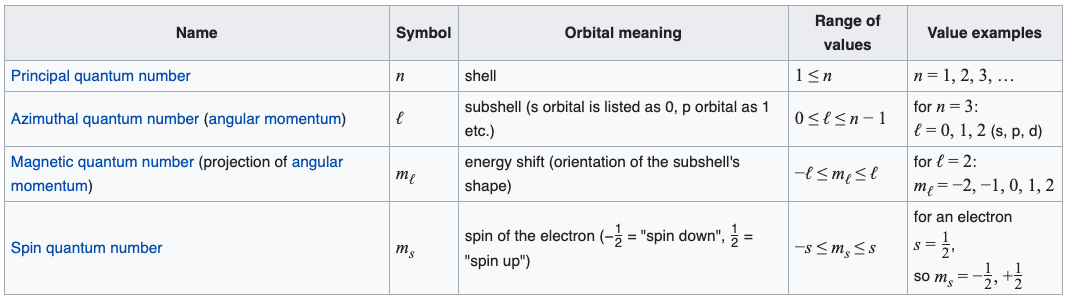
Boston: McGraw-Hill, , p. Thus the angular momentum vectors lie on cones as illustrated. The set of numbers used to describe the position and energy of the electron in an atom are called quantum numbers. For each of these orbitals, there are two allowed values of the spin quantum number, s. Define spin quantum number. One of these orbitals is oriented along the X axis, another along the Y axis, and the third along the Z axis of a coordinate system, as shown in the figure below. Notation for conserved quantities in physics and chemistry. The energy of the subshells gradually becomes larger as the value of the angular quantum number becomes larger. Note that these clouds of probability are the locations of electrons as determined by making repeated measurements—each measurement finds the electron in a definite location, with a greater chance of finding the electron in some places rather than others. Hidden categories: Wikipedia articles needing page number citations from November Wikipedia articles needing page number citations from February Pages with missing ISBNs Articles with short description Short description matches Wikidata Wikipedia articles needing clarification from August Articles with GND identifiers.