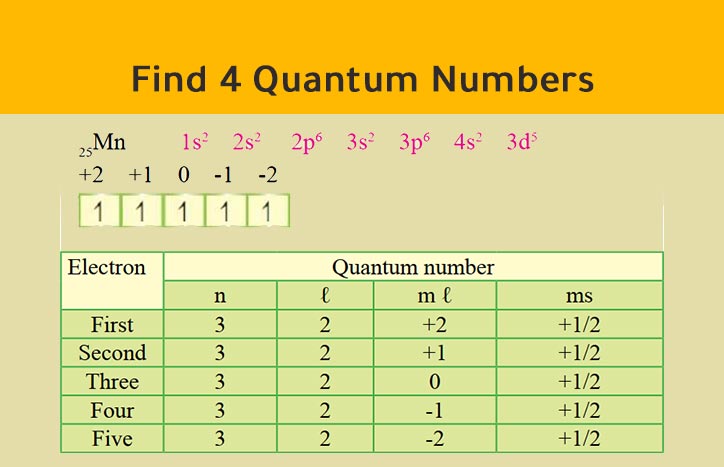
Quantum Numbers (Principal, Azimuthal, Magnetic & Spin) With Examples
Periodic Table with Periods Labeled. A very simple device can be constructed to estimate the relative energies of atomic orbitals. It therefore required three coordinates, or three quantum numbers , to describe the orbitals in which electrons can be found. Heisenberg Uncertainty Principle : According to the Heisenberg Uncertainty Principle, we cannot precisely measure the momentum and position of an electron at the same time. When this happens, the electrons are said to be paired. Within a time of the periodic table the first element introduces a new key energy level. This arrangement is not given in terms of exact positions, like the photograph of a street, but rather in terms of probability distributions and potential energy levels , much like the mosquito swarm.